Flexing A New Strategy for Functional Muscle Regeneration to Heal Large Lesions
Scientists engineer a synthetic scaffold that hosts transplanted muscle progenitor cells to heal severe muscle loss
Research published online in Advanced Materials in February, 2021
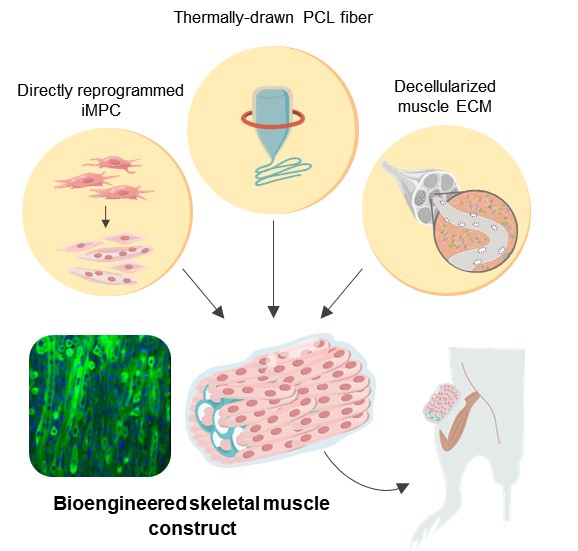
The proposed strategy combines pig-derived muscle extracellular matrix and a biodegradable polymer fiber to create a scaffold for muscle progenitor cells that mimics the 3D microenvironment of muscle. This approach can restore muscle functionality and strength even after critical injury.
Photo courtesy: Yonsei University
Compared with other types of tissue, muscles have remarkable regenerative capabilities. Unfortunately, when a considerable volume of muscle is lost due to severe injury or surgery, the surrounding muscle tissue cannot always repair the damage by itself. One promising treatment strategy that is seeing much research lately is transplanting induced myogenic progenitor cells (iMPCs) into the damaged zone. iMPCs are easily culturable cells that can readily turn into new muscle given the proper conditions and chemical signals.
However, simply transplanting iMPCs does not guarantee a full recovery of muscular function and strength. “To ensure effective muscle regeneration, it is critical to control the microscopic 3D environment and the extracellular matrix (ECM)—which is a scaffold for cells—at the injury site,” explains Prof. Seung-Woo Cho from Yonsei University, Korea. Prof. Cho has been leading a research team with the aim of finding an effective strategy to regenerate muscle using iMPCs with innovative muscle-cell scaffoldings.
In a recent study, Prof. Cho and colleagues from Yonsei University collaborated with scientists from Massachusetts Institute of Technology (MIT), USA, to develop a novel muscle construct containing iMPCs that can ‘patch up’ severe muscle lesions by promoting proper tissue regeneration. Their versatile technique to provide hybrid scaffolding for the iMPCs combines the strengths of both natural and synthetic approaches while accurately mimicking the actual microenvironment found around muscle cells.
The scientists first extracted a large amount of ECM from pig muscle tissue and removed the cells contained within, leaving only an inanimate supporting ‘skeleton.’ However, they knew from previous studies that ECM alone fails to provide the necessary mechanical support for transplanted cells, especially for large lesions. To solve this problem, they carefully engineered synthetic fibrous structures made from polycaprolactone (PCL), a type of biodegradable polymer. Through a chemical process, they created interconnected pores in the PCL fibers, which were able to host ECM with iMPCs.
In experiments with mice, the team demonstrated that their strategy could completely restore muscular structure, strength, and function even after critical injury (75% of muscle lost at the quadriceps). Excited about the results, Prof. Cho remarks that their bioengineering technology could be used to fabricate patient-specific artificial muscles with a wide variety of clinical applications. “Our techniques will hopefully be useful for patients who are suffering from the loss of large muscles due to tumor removal, traumatic injuries, and infectious diseases, as well as elderly people whose muscles are deteriorating due to aging,” he explains. Given the clinical potential of this study, it was selected as the front cover of Advanced Materials, where the paper is published.
Hopefully, some years from now, what would’ve been otherwise regarded as irreparable muscular damage will be considered a completely tractable problem.
A new study by scientists from Korea, which was also selected as the front cover of Advanced Materials proposes a strategy for muscle regeneration that, aside from people who suffered severe muscle loss to injury, surgery, or infection, could also serve the elderly, whose muscles tend to deteriorate owing to aging
Photo courtesy: Wiley Online Library
Recommended Articles
Professor Myeong Min Lee
A QUIRKY twist of fate: understanding epidermis cell differentiation in plants
Professor Jihyun F. Kim
Microbial Mercenaries for Plant Disease Resistance Ungrounded