Three-dimensional liver organoid represents breakthrough for drug screening and tissue engineering
The liver is the largest internal organ and is primarily responsible for the metabolism of drugs. Because of this, it is often the most severely affected by drug toxicity. Scientists have therefore long sought ways of testing the toxicity of drug candidates in liver cells in the laboratory. Adequate sources of liver cells are limited, however. An even bigger challenge is that when cultured outside the body, liver cells are difficult to maintain, quickly losing liver cell-specific features and functions. Two-dimensional (2D) culture conditions can also be vastly different from the three-dimensional (3D) cellular environment inside the liver. Thus, there is some debate over how faithfully 2D-cultured liver cells mirror their counterparts inside the body. Finally, as the growth of liver cells can be time- and labor-intensive, it is difficult to simultaneously screen a large number of drug candidates to determine their toxicity.
In a recent article in Advanced Functional Materials, however, Prof. Seung-Woo Cho and colleagues from Yonsei University and Soongsil University in Seoul, Korea tackle each of these problems, resulting in a breakthrough for the fields of drug development and regenerative medicine: a 3D liver organoid composed of induced liver cells that can be used for high-throughput analyses.
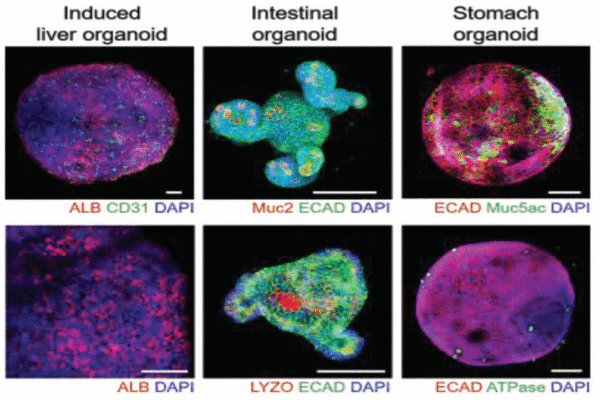
Establishment of a multiorgan model by integrating 3D induced liver tissue with intestinal and stomach organoids in a high-throughput microfluidic device.
The first step in building this system was the development of a 3D scaffold upon which the liver cells could grow. This was achieved by creating a hydrogel out of a slurry of proteins that make up the extracellular matrix, the scaffold upon which liver cells grow in the body. Next, the researchers took cells found in connective tissue known as fibroblasts and inserted a handful of genes normally expressed in the liver, inducing them to transform into liver cells. Cho and colleagues then grew these cells along with the developed scaffold in a 3D microfluidic device capable of generating dynamic flow conditions. Notes Dr. Cho, “Unlike other such devices, which require pumps and tubing to create flow, our microfluidic chamber system is much simpler, relying on a rocker and gravity to create flow in the culture environment.” Such a set-up more easily allows drug candidates to be screened on a large scale.
As a proof of concept, the authors applied their high-throughput 3D liver organoid culture system to simultaneously test the effects of multiple concentrations of the drug acetaminophen on liver cells. The platform was also used to establish a multiple-organ model by integrating the liver organoid with intestinal and stomach organoids, thereby providing a more accurate system for the study of drug metabolism.
The advantages of the new high-throughput 3D induced liver organoid system will make it invaluable for research in multiple fields of biology.
As Dr. Cho notes, the system “provides a renewable source for patient-relevant and powerful high-throughput screening for drug development.” This culture platform may also be useful in cell replacement therapy and liver tissue engineering, helping to treat patients with liver disease.
Updated in Dec 2018
Recommended Articles
Professor Myeong Min Lee
A QUIRKY twist of fate: understanding epidermis cell differentiation in plants
Professor Jihyun F. Kim
Microbial Mercenaries for Plant Disease Resistance Ungrounded